Latest consumer product recalls: Ford F-150 pickup trucks, Trader Joe's soup
Several products have been recalled over the past week for health and safety reasons. Here's the latest recalls from July 26 through August 2 that consumers should know about.
1. Ford recalls over 870,000 F-150 pickups in U.S.
Featured
Ford recalls over 870,000 F-150 pickups in US
Affected trucks could experience sudden activation of their electric parking brakes.
Ford is recalling more than 870,000 newer F-150 pickup trucks in the U.S. because the electric parking brakes can turn on unexpectedly.
The recall covers certain pickups from the 2021 through 2023 model years with single exhaust systems. Ford's F-Series pickups are the top-selling vehicles in the U.S.
Click here for more information
2. Over 10,000 cases of Trader Joe's broccoli cheddar soup recalled due to bugs in florets
Featured
Over 10,000 cases of Trader Joe's broccoli cheddar soup recalled due to bugs in florets
The recalled Trader Joe's soup went to stores in seven states.
A voluntary recall for nearly 10,900 cases of Trader Joe’s broccoli cheddar soup is underway.
Frozen broccoli florets in cases of 20-ounce Trader Joe’s Unexpected Broccoli Cheddar Soup contained insects, prompting the recall, an enforcement report published by the Food and Drug Administration (FDA) said.
Click here for more information
3. Wheels on children's playsets recalled due to fall hazard

(U.S. Consumer Product Safety Commission)
About 3,500 Sky Wheels are being recalled because they can become detached from the overhead rail on the incorporated playset, posing a fall hazard. Officials have received three reports of injuries.
The wheels were sold to various businesses, including McDonald's, Burger King, Lifetime Fitness, and YMCA.
Consumers should contact Soft Play to schedule a free inspection. A repair will be made if needed.
Click here for more information
4. Liquid probiotics recalled for possible contamination

(FDA)
"Ozona Organics, LLC of Ozona, Texas, is recalling its 4-ounce and 16-ounce bottles of Ozona Probiotics for Digestive Health (intended for human use), also labeled as GoHealthy Probiotics for Infants, Toddlers and Kids in 2-ounce bottles and GoHealthy Probiotics for Infants, Kids, Men and Women in 4-ounce bottles because they have the potential to become contaminated with microbial growth," the FDA wrote in a news release.
The company says the products are being recalled "because of high water activity in the formula that provides a potential for microbial growth, which may be harmful."
The products were sold through the company's website, as well as Amazon.
No illnesses have been reported.
The following lot numbers, manufacturing and expiration dates are included in the recall:

(FDA)
Additionally, Ozona Organics Dog, Cat, Equine, and Swine Probiotics are also being recalled. The distribution dates for these products are from August 2021 through July 2023. They were sold on the company's website.
Consumers who have the affected products should throw them away.
Click here for more information
5. Birth control tablets recalled, ‘could potentially result in unexpected pregnancy’

Birth control pills recalled, could reduce effectiveness
Lupin, the company that makes Tydemy, is recalling two lots after tests showed lower levels of vitamin C. The FDA says that can reduce effectiveness and lead to unexpected pregnancies.
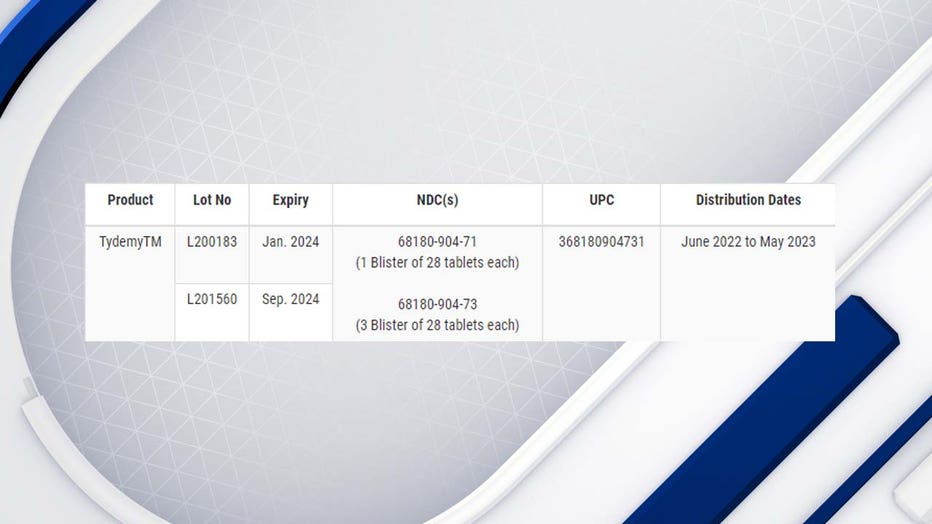
"Lupin Pharmaceuticals Inc. (Lupin) is voluntarily recalling two (2) lots of Tydemy (Drospirenone, Ethinyl Estradiol and Levomefolate Calcium Tablets 3mg/0.03mg/0.451 mg and Levomefolate Calcium Tablets 0.451 mg) to the patient (consumer/user) level due to out of specification (OOS) test results at the 12-month stability time point. Specifically, one lot (L200183) tested low for ascorbic acid (an inactive ingredient) and high for a known impurity," the FDA wrote in a news release.
The products were sold nationwide in drug stores, pharmacies and supermarkets. No "adverse events" have been reported.
"Regardless, Lupin is recalling these two batches because if there were a significant reduction in the amount of inactive content (ascorbic acid), this could potentially impact the effectiveness of the product which could potentially result in unexpected pregnancy," the FDA added.
Consumers taking Tydemy "are advised to continue taking their medication and immediately contact their pharmacist, physician, or medical provider for advice regarding an alternative treatment."
The following table lists the affected product codes:
(FDA)
Click here for more information



