ADHD medication recalled because bottles may contain different drug that has opposite effect

Zenzedi, pictured above, is a stimulant used for the treatment of ADHD and narcolepsy. (Credit: FDA/Azurity Pharmaceuticals)
An ADHD medication was recalled in the U.S. after a different prescription drug was found inside the bottle by a pharmacist, U.S. officials said.
The recall, announced on Jan. 25 in a notice by the U.S. Food and Drug Administration, impacts one lot of 30-milligram dextroamphetamine sulfate tablets sold under the brand name Zenzedi.
Azurity Pharmaceuticals said the product was being voluntarily recalled after a pharmacist in Nebraska reported opening a bottle labeled as Zenzedi 30-milligram tablets and found tablets of carbinoxamine maleate, an antihistamine drug.
"Upon learning of the incident, the manufacturer opened a product complaint and an investigation followed," the recall notice states.
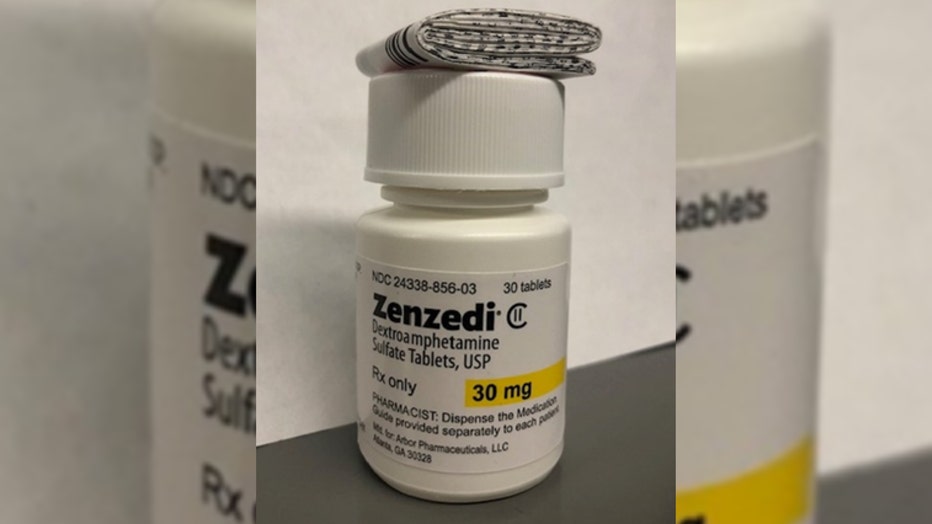
The recalled Zenzedi medication is part of lot number F230169A and expires in June 2025, according to the recall notice. (Credit: FDA/Azurity Pharmaceuticals)
Zenzedi is a stimulant medication used for the treatment of both narcolepsy and ADHD, which is short for attention deficit hyperactivity disorder. Carbinoxamine maleate is used to treat allergies and can have the opposite effect, serving as a sedative in some patients, according to the National Institutes of Health.
"For patients with Attention Deficit Hyperactivity Disorder (ADHD) and Narcolepsy (sleep disorder) there is a reasonable probability that accidents or injuries that occur due to the sedating effects of carbinoxamine, could lead to ongoing disability or death in severe cases, particularly if individuals who use it (unaware that they have not received Zenzedi®) engage in activities requiring significant focus and alertness (e.g., driving, operating heavy machinery)," the FDA notice warns.

FILE IMAGE - A pharmacy technician grabs a bottle of drugs off a shelve at a pharmacy on Sept. 10, 2018, in Midvale, Utah. (Photo by George Frey/Getty Images)
The recalled Zenzedi medication is part of lot number F230169A and expires in June 2025, according to the recall notice. The medication was shipped nationwide between Aug. 23 and Nov. 29, 2023.
The Zenzedi 30-milligram tablets are light yellow and in a hexagonal shape with "30" on one side and "MIA" on the other side and distributed in a white bottle with black writing and "30 mg" highlighted yellow.
Meanwhile, the carbinoxamine maleate tablets, which were provided by the reporting pharmacist, are white round tablets with imprints of "GL" on one side and "211" on the other side, the FDA said.
2019 video: Fetal exposure to painkiller acetaminophen in pregnancy linked to higher risk of ADHD and autism, study says
(2019 video) Fetal exposure to acetaminophen in pregnancy could increase a child’s risk for attention deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD), according to a study.
Azurity Pharmaceuticals said it had not received any serious injury reports related to the recall.
Consumers who have the recalled medication should immediately return it to their pharmacy and contact their health care provider if they have experienced any problems. Problems can be reported to Azurity via email at aereports@azurity.com or the FDA's MedWatch Adverse Event Reporting program.
This story was reported from Cincinnati.

