Moderna begins testing 'next generation' COVID-19 vaccine with potential refrigeration storage
CAMBRIDGE, Mass. - Moderna, a developer behind one of three currently approved COVID-19 vaccines being distributed in the U.S., said it has dosed the first patients in an early-stage study of its "next generation" shot.
The company announced Monday that its new candidate, called mRNA-1283, could potentially be stored in refrigerators instead of freezers making it easier to distribute and administer — particularly in developing countries.
Moderna’s current two-dose vaccine, which received emergency approval by the Food and Drug Administration on Dec. 28, must be stored at minus 20 degrees Fahrenheit, or at normal freezer temperatures. Another approved vaccine being used in the U.S. by Pfizer-BioNTech requires ultra-cold temperatures at minus 94 degrees Fahrenheit.
But nearly 3 billion of the world’s 7.8 billion people live where temperature-controlled storage is insufficient for an immunization campaign to bring COVID-19 under control, according to the Associated Press.
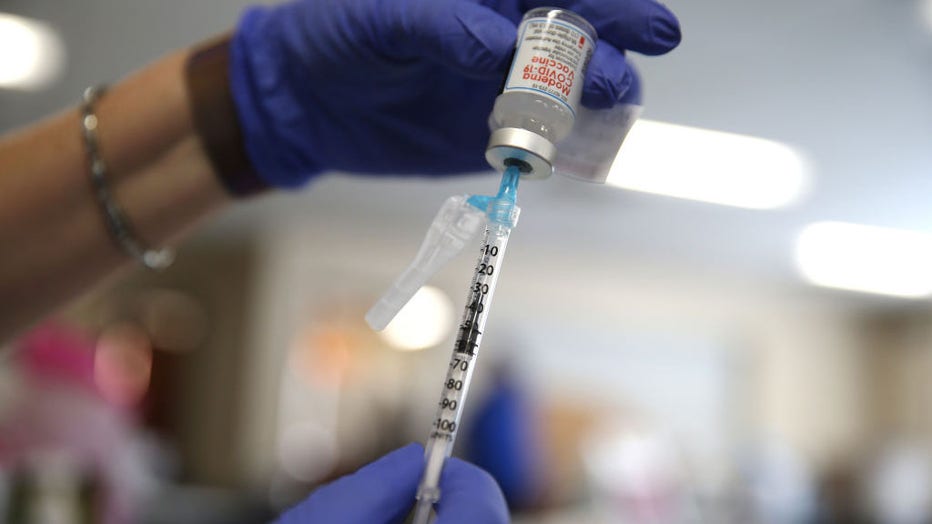
FILE - A nurse prepares a dose of the Moderna COVID-19 vaccine at Lincoln Memorial Congregational Church UCC on March 12, 2021 in Los Angeles, California. (Photo by Mario Tama/Getty Images)
"We are pleased to begin this Phase 1 study of our next generation COVID-19 vaccine candidate, mRNA-1283," said Moderna CEO Stéphane Bancel in a statement. "Our investments in our mRNA platform have enabled us to develop this next generation vaccine candidate, which is a potential refrigerator-stable vaccine that could facilitate easier distribution and administration in a wider range of settings, including potentially for developing countries."
RELATED: Fauci, other top health officials publicly receive first dose of Moderna COVID-19 vaccine
The Phase 1 study will assess the safety of and the immune response generated by mRNA-1283 at three dose levels, the company said. It is being given to healthy adults either as a single dose or in two doses spaced 28 days apart.
Both Moderna’s and Pfizer-BioNTech’s shots are mRNA vaccines, made with groundbreaking new technology. They don’t contain any coronavirus – meaning they cannot cause infection. Instead, they use a piece of genetic code that trains the immune system to recognize the spike protein on the surface of the virus, ready to attack if the real thing comes along.
A third single-dose vaccine by Johnson & Johnson has also been approved for use in the U.S. — helping to ramp up vaccine supply as the country works to stem the virus that has killed nearly 535,000 Americans.
The U.S. has administered more than 100 million doses of COVID-19 vaccine, averaging about 2.2 million vaccine doses administered per day. President Joe Biden has declared that all adults should be eligible for coronavirus vaccinations by May 1.
RELATED: France, Germany latest countries to suspend AstraZeneca COVID-19 vaccine amid blood clotting reports
This story was reported from Cincinnati. The Associated Press contributed.

